INTRODUCTION
Sexing of chicks plays a crucial role in the economic aspects of the poultry industry. It is impossible to visually distinguish between male and female chicks based on their appearance immediately after hatching. Therefore, numerous studies have been conducted to identify the sex of chicks. Recently, research on ovo-sexing has been actively pursued. Techniques involving the analysis of sex steroid hormones, such as estradiol or its metabolite estrone sulfate, in embryos were proposed for pre-hatch sex identification (Weissmann et al., 2013). Another approach employed fluorescence and Raman spectroscopy for sex identification from embryo blood samples (Galli et al., 2018). However, these methods faced challenges in industrial applications. Consequently, post hatched chick sexing methods are commonly employed. The representative methods for sex identification of hatched chicks are vent-sexing (Masui and Hashimoto, 1933) and feather-sexing based on sex-linked inheritance (Gawron and Smyth, 1980; Campo, 1991; Pal and Singh, 1997; Sohn et al., 2012). Among the feather-sexing methods, the feather discrimination method utilizing characteristics associated with the rate of feather development, such as early-feathering and late-feathering traits, is the most widely used. This method involves mating late-feathering (ZKW) maternal chickens with early-feathering (Zk+Zk+) paternal chickens, resulting in female offspring inheriting early-feathering (Zk+W) and male offspring inheriting late-feathering (ZKZk+). This distinction enables sex identification through feather morphology (Somes, 1969; Iraqi and Smith, 1995; Bang et al., 2018; Qiu et al., 2022). However, the feather morphology of late-feathering and early-feathering individuals can vary depending on the breed and strain. Furthermore, three days after hatching, the feather pattern becomes similar among individuals, making it difficult to distinguish between late-feathering and early-feathering individuals (Siegel et al., 1957; Sohn et al., 2012; Bang et al., 2018). Therefore, research has been conducted to identify genes associated with feather development and utilize the related genes to determine the sex of chicks. It was known that the late-feathering specific gene K was located on the Z chromosome and was associated with the Avian ev21 gene insertion site, as well as with the prolactin receptor gene PRLR and the sperm head protein gene SPEC2 (Bacon et al., 1988; Boulliou et al., 1992; Elferink et al., 2008; Luo et al., 2012; Zhao et al., 2016). Consequently, it was reported that the use of the ev21 gene for PCR allowed for straightforward identification of late-feathering chickens (Iraqi and Smith, 1994). However, there were also reports suggesting that the ev21 gene was not responsible for the expression of late feathering in all chicken breeds (Bu et al., 2013; Zhao et al., 2016; Takenouchi et al., 2018; Okamura et al., 2019; Shen et al., 2023). Therefore, in this study, we introduced the new sequences of ev21 related late-feathering specific locus for breed-independent identification of late-feathering chickens. We also investigated the genetic transmission pattern of the identified sequences.
MATERIALS AND METHODS
To investigate the late-feathering gene, we employed a total of 707 chickens including Single Comb White Leghorn, Black Cornish, Rhode Island Red, Korean Ogye, Korean Native Chicken breeds for our research. These chickens were raised in pullet battery cages (8,100 cm2/cage) with a stocking density of 810 cm2 per bird for the initial 10 weeks of their growth period. Temperature, humidity, and lighting management were artificially controlled throughout this period. Subsequently, the chickens were transferred to floor pens, which were spacious at 25.5 m2 per pen, with a stocking density of 2,550 cm2 per bird. During this period, the chickens were provided with restricted feeding until they reached 60 weeks of age. The chicks were classified as either early-feathering or late-feathering based on their feather development immediately after hatching. The care and handling of these chickens adhered to the guidelines established by the Institutional Animal Care and Use Committee (IACUC, No. 2020-5) of our university.
Feather pulp cells and blood cells were collected from chickens to prepare the samples. Feather pulp cells were prepared from the wing feathers of chicks aged 2 to 3 days, while whole blood was obtained from the brachial wing vein at both 12 and 35 weeks of age, using standard blood collection procedures. Genomic DNA was subsequently extracted from the feather pulp cells and blood cells using the High Pure PCR Template Preparation Kit (Roche, Mannheim, Germany).
Based on the close linkage between the endogenous retrovirus ev21 gene and the feather development gene in chickens, we designed primers specific to the ev21-related late-feathering gene. The primer sequences are as follows; Forward 5’-GGGGTCAGCATGTTTAAAGGA-3’and Reverse 5’-GTGCACCTGGGTGTAGATGG-3’. PCR reactions were prepared using 2.5 μL of 10× buffer, 2 μL of a dNTP mixture (2.5 mM), 2 μL of primers (5 pmol/μL), and genomic DNA (100 ng/μL) in a total reaction volume of 25 μL (TaKaRa, Tokyo, Japan). The cycling conditions comprised an initial denaturation step of 5 minutes at 95°C, followed by 32 cycles at 95°C for 1 minute, 56°C for 30 seconds, and 72°C for 1.5 minutes. The resulting PCR amplification products were electrophoresed on a 1.2% agarose gel at 120V for 20 minutes and were confirmed as early- or late-feathering based on the presence or absence of specific bands. Furthermore, the PCR amplification products were sequenced (Macrogen Inc., Seoul, Korea), and the nucleotide sequences were compared with the NCBI Blast database (National Center for Biotechnology Information).
To investigate the genetic transmission patterns of the ev21-related K specific sequences, various mating combinations were established between early-feathering (EF) and late-feathering (LF) chickens. The distribution ratio of EF and LF in the offspring, along with their genotypes, was analyzed. The mating combinations included LF (♂) × EF (♀), LF (♂) × LF (♀), EF (♂) × LF (♀), and EF (♂) × EF (♀). Genetic transmission patterns were examined for approximately 20 offspring from each of the five families for each combination.
RESULTS
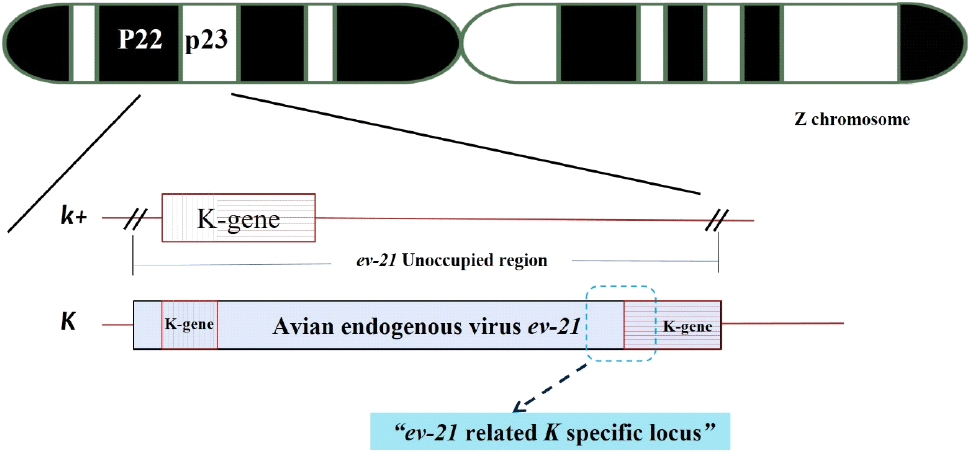
To identify a late-feathering specific gene for distinguishing between early-feathering and late-feathering chickens, we conducted an exploration of the ev21-related locus. The targeted loci included the Avian leukosis virus (ev21) gene locus, spanning 9,679 bp on the Z chromosome (GenBank: KY235336.1), and the K-gene locus related to feather development, spanning 1,444 bp (GenBank: AB610980.1). As the K-gene and ev21 gene are closely related, a comparative analysis of their homology revealed a 99% match in the 1 to 142 bp of the K-gene with the 835 to 976 bp of ev21, and a 98% match in the 136 to 1,312 bp region of the K-gene with the terminal 8,500 to 9,679 bp of ev21. Therefore, we identified only the late-feathering related specific locus from the ev21 gene in chickens (GenBank: KY235336.1) to create a 230 bp of ev21-K locus. Sequence analysis revealed similarities of 98.7% with the ev21 gene of the Huxu breed, 63.9% with USIL-1, and 65.7% and 67.4% with the K-gene of the Geline and Nagoya breeds, respectively. It also showed 98.7% similarity with the JFIL-1 gene, as presented in Table 1. According to this structure, the presence of the ev21 gene indicates late-feathering chickens, while its absence indicates early-feathering chickens. Consequently, this study identified sequences specifically present in late-feathering chickens, focusing on the overlapping region of the terminal portion of the ev21 gene and the K gene. The identified sequences were named the ev21-related K specific sequences, with a determined size of 230 bp. Its DNA sequence is presented in Fig. 1, with a schematic representation of the structure in Fig. 2. To confirm if the ev21-related K specific sequences are presented exclusively in late-feathering chickens, PCR was performed using ev21-related K specific DNA primers on early-feathering and late-feathering chickens confirmed through phenotypical feather-sexing. The analysis results, as shown in Fig. 3, confirmed that all late-feathering chickens exhibited a clear band at the 230 bp position, while no band was observed in early-feathering chickens. This observation confirmed that the identified sequences serve as a specific DNA marker presented only in late-feathering chickens.
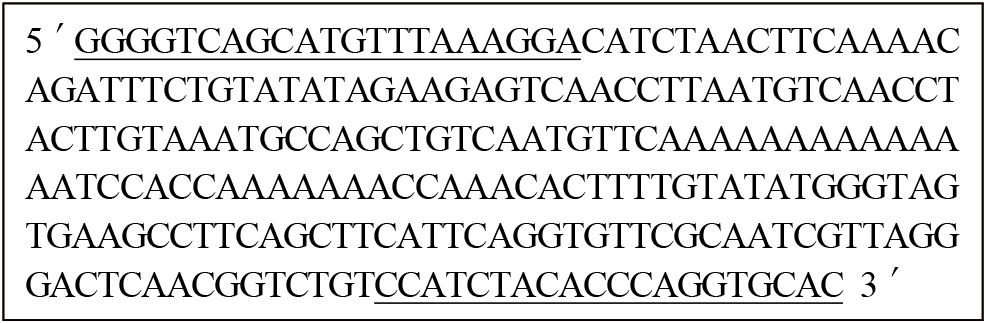
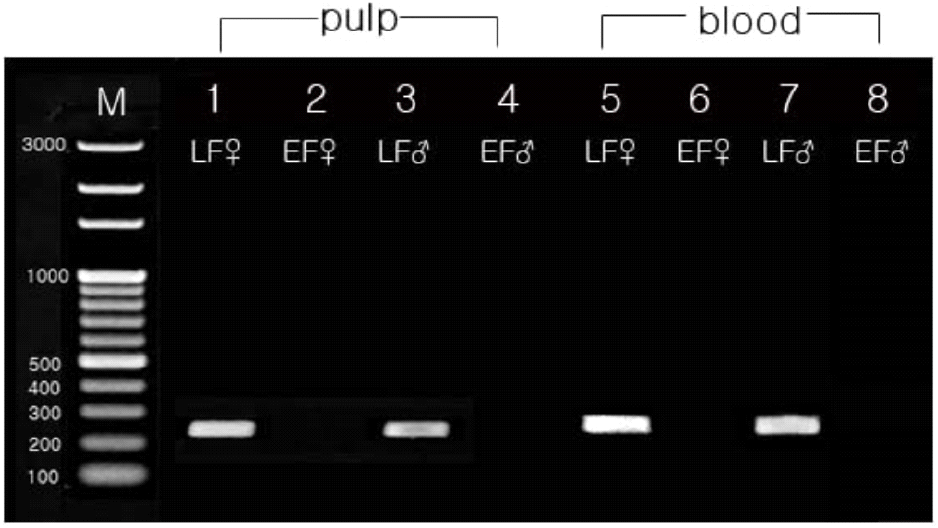
To assess the reliability of using the ev21-related K specific sequences to identify late-feathering chickens, comparative analyses were conducted across various factors, including tissues, age groups, and breeds. Tissue-specific comparisons were made using feather pulp cells obtained from feather tissue and lymphocytes obtained from blood. The PCR amplification products of the ev21-related K specific sequences were compared and analyzed between these two sample types. As shown in Fig. 3, late-feathering chickens showed a distinct band of 230 bp in both feather pulp cells and blood cells, and early-feathering chickens did not show a band in either cell type. Additionally, to verify the presence of the ev21-K specific sequences in different breeds, PCR analysis was performed on the Korean Native Chicken-Red and -Yellow strains, White Leghorn, Black Cornish, Rhode Island Red, and Korean Ogye. Based on feather-sexing results, Korean Native Chicken-Red strain, Black Cornish, and Rhode Island Red exhibited both early-feathering and late-feathering chicks, while Korean Native Chicken-Yellow strain, White Leghorn, and Korean Ogye were exclusively identified as early-feathering chicks. The consistency between feathering phenotypes and PCR results for each breed was analyzed, and the findings were presented in Table 2. The analysis revealed a 100% match between feathering phenotypes and PCR results in all breeds, except for one late-feathering chicken out of 54 in the Korean Native Chicken-Red strain. To investigate whether ev21-related K specific sequences exhibit consistent effects across different ages, chickens at 12 and 35 weeks of age were subjected to PCR testing using lymphocytes. The PCR results by age group showed consistent findings at both 12 and 35 weeks of age across all three breeds, as shown in Table 3.
To investigate the genetic transmission patterns of the ev21-related K specific sequences, crossbreeding tests were conducted between the early-feathering (EF) and late-feathering chickens (LF). The presence or absence of the ev21-related K specific sequences in their offspring were examined, and the results were presented in Table 4. In the crossbreeding test results, both individuals with and without the ev21-related K specific sequences were observed in chicks produced by LF (♂) × EF (♀) crossbreeding. Additionally, in LF (♂) × LF (♀) crossbreeding, only chicks with the ev21-related K specific sequences were observed among the male progeny, while both individuals with and without the sequences were observed among the female progeny. On the other hand, all male progeny produced by EF (♂) × LF (♀) crossbreeding had the ev21-related K specific sequences, while none of the female progeny had the sequences. In the case of EF (♂) × EF (♀) crossbreeding, none of the progeny had the ev21-related K specific sequences. The distribution and frequencies of the ev21-related K specific sequences in the chicks produced from several crossbreeding combinations were found to be consistent with the phenotypic patterns determined by feather-sexing, showing a 100% match.
DISCUSSION
The avian leukosis virus (ALV) is categorized as a retrovirus, with distinctions between exogenous and endogenous viruses (Payne, 1998; Mo et al., 2022; Yang et al., 2022). Within ALV subgroups, ALV-E is recognized as a non-pathogenic or low-pathogenic endogenous retrovirus in chickens. The ev insertion locus on the Z chromosome is closely linked to the late-feathering gene K (GenBank: AB610980.1), and the prolactin receptor (PRLR) gene (Johnson and Heneine, 2001; Luo et al., 2012; Su et al., 2018; Mays et al., 2019; Borodin et al., 2022). The presence or absence of the ev21 gene allowed for the distinction between early-feathering and late-feathering, making it a molecular marker for identifying late-feathering chickens (Bacon et al., 1988; Iraqi and Smith, 1994; Smith and Fadly, 1994; Tixier-Boichard et al., 1994; Zhang et al., 2018). However, recent studies have reported that the ev21 gene may not be an absolute marker for identifying late-feathering chickens (Elferink et al., 2008; Bu et al., 2013; Zhao et al., 2016; Takenouchi et al., 2018; Okamura et al., 2019; Shen et al., 2023). Levin and Smith (1990) reported the loss of the ev21 insertion site but the presence of the ev21 gene in DNA analysis of early-feathering revertant females. Takenouchi et al. (2018) confirmed the absence of the ev21 gene in late-feathering chickens of the Ingie breed by analyzing the genotypes of 52 chicken breeds using qRT-PCR. Wimmers et al. (1996) also reported that some late-feathering birds possess a structural variant of ev21, in which major parts of ev21 are missing. Furthermore, Boulliou et al. (1992) and Tixier-Boichard et al. (1994) pointed out the existence of early-feathering birds that have ev21. Based on these results, the ev21-related K specific sequences are considered a late-feathering specific marker regardless of the ev21 gene region of chickens. Comparative analyses conducted among tissues, breeds, and ages confirmed the utility of identifying late-feathering chickens using the ev21-related K specific sequences. The results of inter-tissue comparisons indicated applicability to all tissue cells, with consistent outcomes among tissues from the same chicken. Additionally, as it was specifically presented in late-feathering chickens regardless of the breed, it was confirmed to be a useful molecular biological marker for late-feathering identification. Moreover, the identification of late-feathering chickens using the ev21-related K specific sequences are applicable from hatched chicks to adult chickens, making it beneficial for the establishment of feather-sexing strains. Through various crossbreeding tests between early-feathering and late-feathering chickens, we examined the genetic transmission patterns of the ev21-related K specific sequences to the next generation. The results showed that offspring in all crossbreeding combinations exhibited phenotype segregation patterns according to sex-linked inheritance, and the transmission patterns of the ev21-related K specific sequences were consistent with the segregation patterns of the phenotype. Furthermore, the location of the ev21 gene on the sex-linked Z chromosome and its close association with the late-feathering gene K have been reconfirmed. In the LF (♂) × EF (♀) crossbreeding test, both early-feathering and late-feathering chicks were produced in all families, indicating that the LF paternal is presumed to have a heterozygous genotype of ZKZk+. On the LL4 and LL5 families in LF (♂) × LF (♀) crossbreeding, all male and female offspring were late-feathering chicks, indicating that the LF paternal is presumed to have a homozygous genotype of ZKZK. In the EF (♂) × LF (♀) combination, the fact that all females of the produced chicks were early-feathering and all males were late-feathering indicated the possibility of auto-sexing by feather morphology of chicks. In conclusion, the newly identified ev21-related K specific sequences are a robust and reliable molecular marker for distinguishing between early-feathering and late-feathering chickens, independent of breed and age. The observed genetic transmission patterns of the ev21-related K specific sequences align with sex-linked inheritance, affirming its potential as an inheritable marker for late-feathering traits. Identification of the ev21-related K specific sequences are a useful tool for feather-sexing, and can greatly aid in the construction of auto-sexing lines in the poultry industry.
SUMMARY
본 연구는 조우성과 만우성 닭을 식별하기 위한 유전자 마커를 발굴하고자 한 것으로 만우성과 관련된 ev21-K라는 새로운 좌위를 발견하고 이의 특성을 구명하였다. 더불어, 본 좌위의 유전적 전이 양상을 조사하여 자가 성감별 라인 조성의 이용 가능성도 살펴보았다. 본 시험을 위해 5개 품종의 닭 707수를 공시하고 이를 대상으로 유전자 마커 발굴 및 유전적 전이 시험을 수행하였다. ev21-K 특이 좌위 발굴은 깃털 발육과 연관된 ev21 유전자와 만우성 유전자인 K 유전자를 탐색하고 이들 간 염기서열을 비교 분석하여 획득하였다. 확인된 좌위의 분석을 위해 대상 서열에 대한 특정 프라이머를 제작하고 중합효소연쇄반응(PCR)을 수행하여 결과물을 획득한 후 이들의 염기 서열을 분석하였다. 발굴된 염기 서열의 유전적 전이 양상을 조사하기 위하여 조우성과 만우성 닭 간의 교배조합시험을 수행하였다. 시험 결과, 발굴된 230 bp ev21-K 유전자 좌위를 ev21-related K specific sequences라 명명하였고, 이는 기존 ev21 유전자와 99%의 상동성을 나타내었다. PCR 분석을 통해 해당 서열이 만우성 닭에만 존재하는 것으로 확인되었다. 본 서열은 조직, 품종 및 연령에 관계없이 만우성 닭에만 존재하는 일관된 결과를 보여주었다. 교배 시험을 통하여 본 서열의 전이 양상을 살펴본 결과, 반성 유전을 하며 깃털 표현형과 일치하는 분리 결과를 보였다. Ev21-related K specific sequences의 유전적 전이 양상은 본 서열이 우성으로써 전형적인 멘델 유전에 따르는 것으로 나타났다. 결론적으로, ev21-K 특이 좌위의 새로운 서열은 품종에 관계없이 조우성과 만우성 닭을 식별하기 위한 신뢰할 수 있는 분자 마커로 확인되었다.








