INTRODUCTION
Growing concerns about animal welfare globally, interest in litter management has increased in the broiler industry. Inappropriate management causes poor litter quality, which is one of the main factors in increasing the prevalence of footpad dermatitis (FPD) in broilers (Garcês et al., 2013). The FPD, also known as footpad lesions, is a skin condition problem that is characterized by inflammation, necrotic lesions, and hyperkeratosis ranging from the plantar surface of the footpads and toes (Shepherd and Farichild, 2010). The FPD could induce impaired walking strength through a painful foot skin condition with synovitis and subsequent lameness, which decreased the eager to go to the feeder and drinkers (Clark et al., 2002; Kjaer et al., 2006; Michel et al., 2012).
Generally, litter is a major route for broilers to get exposed to bacterial pathogens through their pecking and coprophagic behavior after broiler placement (Oladeinde et al., 2023). Prolonged contact with bacterial pathogens decreases the structural integrity of skin tissues, which results in an increased prevalence of FPD in broilers (Manangi et al., 2012; Vieira et al., 2013). The FPD is not only caused by bacterial pathogens, and it is believed to be caused by a combination of litter moisture and nitrogen content (Mohamed Amer, 2020). Excessive litter moisture accelerates the volatilization of ammonia from the microbial metabolism in the excreta, resulting in increased bacterial pathogen activities and lesions with FPD in the broiler (Garcia et al., 2012). Also, nitrogen in litter could be converted to ammonia under anaerobic conditions and to nitrate under aerobic conditions (Madigan et al., 1997). Although the replacement of poor litter (wet and high nitrogen) with dry litter could recover the FPD in about 2 weeks, it is not practical or economically likely to replace litter material frequently (Chen et al., 2016). Therefore, alternative strategies should be considered to reduce litter moisture and nitrogen content.
Illite is a non-expanding, clay-sized mineral mixture that contains phyllosilicate or layered aluminosilicate (Sarker and Yang, 2010). Illite forms a large surface area by tetrahedra silica sheets, which improves the water absorption capability (Hatch et al., 2012). Also, illite could absorb water into the interlayer spaces due to its interlayer cations (McConville and Lee, 2005; Choi et al., 2009). Thus, illite (0.6%−1.0%) has been used to reduce pathogenic microorganisms and improve resistance against Salmonellosis through its moisture absorption capability (Lee et al., 2009; Biswas et al., 2018; Lim et al., 2022).
Zeolites are crystalline, hydrated aluminosilicates of alkali and alkaline earth cations that form an infinite, open three-dimensional structure (Noori et al., 2006). The microporous crystalline structure of zeolites makes it possible to adsorb materials that fit through surface entry channels (Turan et al., 2008). Supportably, zeolites showed significant water absorption capability through their three-dimensional network of hydrophilic polymers (Yan et al., 2014; Zadeh et al., 2019). Therefore, numerous studies have been conducted to decrease litter moisture content by using illite and zeolite in broilers (Schneider et al., 2017; Chung and Choi, 2019).
However, most studies focused on the dietary effects of illite and zeolite, which decrease litter moisture and nitrogen content with improved growth performance in broilers (Safaeikatouli et al., 2011; Schneider et al., 2016; Abdelrahman et al., 2023). Moreover, there are only few studies about identifying the effects of spraying illite and zeolite in rice husks as litter (Chung and Choi, 2019). Therefore, the main objective of this study was to investigate and compare the effects of spraying illite and zeolite on litter quality, litter microflora, and FPD.
MATERIALS AND METHODS
The protocol for this study was reviewed and approved by the Institutional Animal Care and Use Committee of Chungbuk National University, Cheongju, Korea (approval no. CBNUA-2107-23-01).
The composition of illite is SiO2, Al2O3, K2O, Fe2O3, Na2O, TiO2, MgO, CaO, P2O5, MnO, respectively, 67.4%, 20.3%, 5.50%, 2.35%, 0.54%, 0.27%, 0.24%, 0.04%, 0.04%, and 0.01%, provided by YonggungIllite (Seoul, Korea). Zeolite is composited with SiO2 and moisture, respectively, 63.23 % and 8.19%, provided by Haedameun (Eumseong, Korea).
A total of 192 one-day-old Ross 308 broilers (initial body weight of 31.30±0.41 g) were obtained from a local hatchery (Dongsan hatchery, Cheonan, Korea) and used in this experiment for 28 days. All broilers were randomly allocated into four treatments in a randomized complete block design. Treatments were as follows: basal rice husk (CON), rice husk + 1% illite (calculated on a weight of litter; T1), rice husk + 1% zeolite (calculated on a weight of litter; T2), and rice husk + 0.5% illite + 0.5% zeolite (calculated on a weight of litter; T3). Each treatment had four replicates, with 12 birds per pen (W: 173 cm, D: 63 cm, H: 55 cm). Each pen was provided with 5 kg of rice husk as litter. Illite and zeolite were sprayed on the surface of the litter and dispersed by using a spreader at the beginning of the experiment. The experiment initiation temperature was 34±1°C, and after that, the temperature was gradually lowered to maintain 25±1°C. The lighting schedule was 23L:1D at 100 lux on d 1, 12L:12D at 30 lux on d 4 until week 2, and 8L:16D at 30 lux thereafter. All diets were formulated to meet or exceed National Research Council (1994) for the starter (1−7 d), grower (8−21 d), and finisher (22−28 d) periods. All broilers were given ad libitum access to diet and water throughout the experiments.
A litter sample was collected weekly at 5 random locations from each pen. In each pen, 5 sampling points were identified: 2 points at the front (in the proximity of the feeders and drinkers), 2 points at the back (away from the feeders and drinkers), and 1 point in the center. The random litter samples were thoroughly mixed, and 100 g was weighed into a plastic bag and refrigerated at 4°C until the samples were analyzed.
To analyze moisture content, 2 g of litter samples from each pen were dried in an oven at 105°C for 8 h according to AOAC (2005). Nitrogen content was determined by the Dumas method (Jung et al., 2003) using a Vario EL Cube (Elementar Analyse System GmbH, Hanau, Germany).
To analyze the counts of litter microflora, litter samples were collected weekly in conical tubes. From the sample, 0.1 g was suspended in distilled water, homogenized, and diluted from 10−4 to 10−7 to count the number of bacteria. Evenly spread 100 μL of the diluted solution on the agar. Escherichia coli (E. coli) and Salmonella were analyzed for bacteria, and MacConkey agar (MB cell, Seoul, Korea) was used for E. coli, and BG Sulfa (MB cell, Seoul, Korea) agar was used for Salmonella.E. coli and Salmonella were cultured for 24 hours at 37°C.
Footpad dermatitis was scored in all birds at the end of the experiment according to the type of lesion according to the Eichner et al. (2007) method. After euthanizing broilers, footpad lesions were scored on a scale from: no lesion (score 0), a lesion covering less than 25% of the sole (score 1), a large area lesion covering between 25% and 50% of the sole (score 2), and more than 50% of the plantar (score 3). Scores were assessed on both paws of the birds, and the raters were independently conducted by three observers. The average score for foot lesions was performed by turning the statistics.
All data were statistically processed using the one-way ANOVA using JMP Pro 16 (SAS Institute, Cary, NC, USA), using each pen as the experimental unit. Differences among all treatment means were determined using the Tukey multiple-range test. The level of significance was established at P<0.05.
RESULTS
As shown in Table 1, litter moisture content was significantly decreased (P<0.05) in the T1, T2, and T3 groups compared with the CON group at week 4. In litter nitrogen, T1 group showed significantly lower (P<0.05) litter nitrogen content than the other groups at weeks 1, 2, and 3. In addition, T3 group showed a significantly lower (P<0.05) litter nitrogen content than the CON and T2 groups at weeks 2 and 3.
Each treatment was provided 5 kg of rice husk as a litter.
The percentage of illite and zeolite was calculated on a weight of litter.
As shown in Table 2, the counts of E. coli in the litter were significantly decreased (P<0.05) in the T1 group compared to the CON group at weeks 2 and 3. Also, the counts of Salmonella in the litter were significantly decreased (P<0.05) in the T1 group than CON group at week 4.
Each treatment was provided 5 kg of rice husk as a litter.
The percentage of illite and zeolite was calculated on a weight of litter.
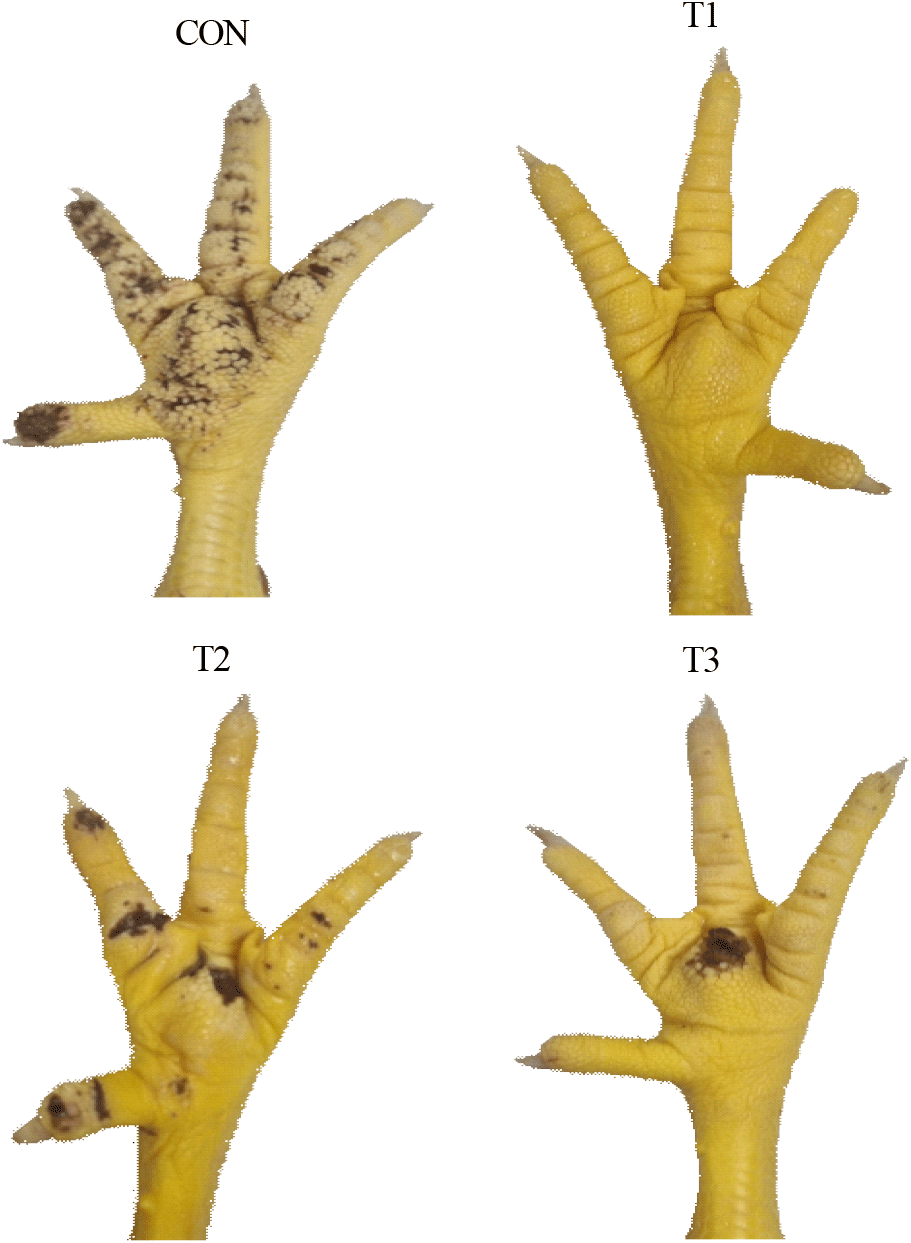
As shown in Table 3, the FPD score significantly decreased (P<0.05) in the T1 group compared to the CON group. Also, illustration of footpad-dermatitis of each treatment were presented in Fig. 1.
| Score2 | CON | T1 | T2 | T3 | SE | P-value |
|---|---|---|---|---|---|---|
| Average | 1.88a | 1.42b | 1.63ab | 1.60ab | 0.083 | 0.002 |
The percentage of illite and zeolite was calculated on a weight of litter.
1 CON, basal rice husk; T1, rice husk + 1% illite; T2, rice husk + 1% zeolite; T3, rice husk + 0.5% illite + 0.5% zeolite; SE, standard error.
2 Lesion score: Lesion score was determined as follow: 0, no lesion; 1, lesion covering less than 25% of the sole of the foot large area lesion; 2, covering between 25% and 50% of the sole of the foot; 3, more than 50% of the lesion of the plantar.
2 Each treatment was provided 5 kg of rice husk as a litter.
DISCUSSION
Litter moisture content is considered as an index of litter quality (Oluwaseyi, 2016). When continuous litter moisture contacts the skin, it softens the tissue and opens the collagen matrix of the epidermis, and it might facilitate the ingress of substances that initiate an immune response (Mayne et al., 2007). Also, it is well documented that continuous exposure to wet litter significantly changes the morphology of the skin, causing pathological responses (Wu and Hocking, 2011).
In the current study, spraying illite and zeolite decreased litter moisture content by 10.54% to 11.14% compared to non-sprayed litter moisture content. Previous studies have reported that using aluminosilicates decreased litter moisture content by its high water-absorbing capabilities in broilers (Safaeikatouli et al., 2011; Gilani et al., 2016). Illite forms large specific areas, layered, and porous molecule structures, which increase the contact area with oxygen and water (Sarker and Yang, 2010). An increased contact area could provide higher aeration and an improved ventilation rate, which induces the evaporation of litter moisture content (Liu et al., 2007; Meng et al., 2021). Correlated with this study, previous studies have reported that spraying zeolite (250 g/kg and 10% of litter total weight, respectively) in litter decreased the 4%−9% of litter moisture content compared to non-sprayed litter (Eleroğlu and Ylacin, 2005; Schneider et al., 2016). Zeolites absorb the water and cations through their specific porous structure, where moisture is captured (Coombs et al., 1997). According to the García (2010) study, zeolite could reduce the litter moisture content due to its reversible capacity for water absorption and it release into the atmosphere by evaporation. Therefore, the observation of a decrease in moisture content by spraying illite and zeolite might be attributed to the water absorption capabilities of illite and zeolite.
Over time, nitrogen accumulates in the litter due to broiler excreta, which is composed of feces and urine (Vilela et al., 2020). Nitrogen is constantly transformed by changes in bacterial activity, temperature, pH, and moisture in litter, and most of the percentage (40%−90%) is converted to ammonia in litter (Kelleher et al., 2002). Consequently, converted ammonia results in impaired litter quality and promotes the development of FPD (Choi and Moore, 2008; Stojčić et al., 2016). In the current study, spraying 1% of illite decreased the litter nitrogen content. This result is in agreement with Slamova et al. (2011), who reported that illite decreased the litter nitrogen content by absorption of ammonium ions due to their cation-exchange capacity and hydration properties. Also, Zhang et al. (2016) have reported that absorbents with a large surface area and cation exchange capacity could decrease litter nitrogen content, which is consistent with illite structure. Moreover, previous studies have reported that litter moisture and ammonia could be converted to ammonia-N by urea hydrolysis and microbial breakdown of uric acid in litter (Martins et al., 2013; Khosravinia et al., 2015). These authors suggest that reducing litter moisture content could decrease conversion to ammonia-N, which results in decreased litter nitrogen content. Therefore, the capabilities of illite in reducing litter moisture and absorbing ammonium ions might be reasonable for reducing litter nitrogen content in this study.
The most concerning point in litter is bacterial pathogens, such as E. coli and Salmonella, which could disseminate to broilers (Ruiz-Barrera et al., 2020). E. coli and Salmonella could exert metabolic activities in the litter, which cause reduced growth and impaired walking strength in poultry (Soliman et al., 2018). In the current study, spraying 1% of illite decreased the counts of E. coli and Salmonella, along with the litter moisture content. The exact mechanism of decreased counts of E. coli and Salmonella by spraying illite has not been previously documented. However, we guessed that a reduction in the counts of E. coli and Salmonella might be attributed to a decrease in litter moisture content. Litter moisture content is one of the major factors for E. coli and Salmonella to survive in litter (Şekeroğlu et al., 2013). Excessive litter moisture content creates favorable conditions for the multiplication of enteric pathogens such as E. coli and Salmonella (De Rezende et al., 2001). In contrast, when the litter moisture falls outside the optimum range for microbial growth and survival, it causes cellular damage and cell death (Soliman et al., 2009). According to Chang et al. (2020), increased counts of Salmonella and E. coli were observed when the litter moisture content induced from 17.78% to 54.34%. Consistently, previous studies have demonstrated that the survival and proliferation rates of E. coli and Salmonella increase as litter moisture content increases (Cools et al., 2001; Wilkinson et al., 2011). Thus, decreased counts of E. coli and Salmonella might be reasonable due to the reduced litter moisture content in this study.
In the current study, spraying 1% of illite in broiler litter caused a significantly decreased FPD score compared to the non-supplementation of illite. This result is in line with the study of Banaszak et al. (2020), who reported that spraying 1% of aluminosilicates (4.50 kg/m2) decreased the FPD score in broilers. The main cause of FPD is litter ammonia, which is produced by moisture and nitrogen, dissolves in wet litter to create an irritant an alkaline solution for the footpads (Bilgil et al., 2009). Consequently, broilers contact with irritant alkaline solution, which causes “ammonia burns”, a factor in FPD (Berg et al., 2004; Jacob et al., 2016). As we mentioned above, previous studies have reported that reducing litter moisture and nitrogen content decreased FPD scores, which is consistent with our results (Youssef et al., 2011; Da Costa et al., 2014; Taira et al., 2014). Moreover, previous studies have demonstrated that decreased litter pH reduced the FPD with diminished litter moisture and nitrogen content (Kaukonene et al., 2016; Stojčić et al., 2016; Zikic et al., 2017). According to the Toledo et al. (2020), litter pH could reduce the litter ammonia content by diminishing free ammonia without charge and the form of ammonium ions in the litter. Therefore, the decrease of FPD score in this study might be reasonable due to reduction of litter moisture and nitrogen by spraying 1% illite.
SUMMARY
In the current study, we found that spraying illite and combination of illite and zeolite increased litter quality. In particular, spraying 1% illite decreased the counts of pathogenic bacteria counts, and FPD scores compared to CON treatment. In conclusion, spraying illite could be an ideal way to improve litter quality and decrease FPD in broilers.








